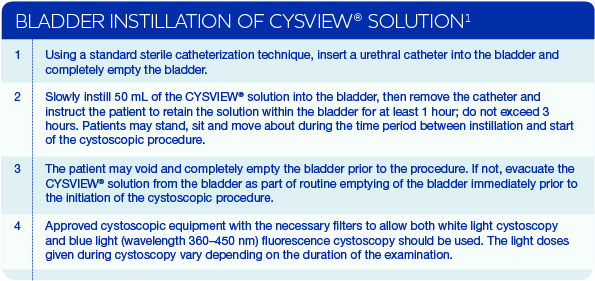
THE PROCEDURE

Blue Light Cystoscopy (BLC®) with Cysview® is indicated as an adjunct to White Light Cystoscopy (WLC) in the detection of papillary non-muscle invasive bladder cancer (NMIBC) in patients with known or suspected bladder cancer.1
Cysview is an optical imaging agent that makes cancer cells fluoresce red/pink in blue light. Blue Light Cystoscopy with Cysview can be performed using only approved cystoscopic equipment with the necessary filters to allow examination using both white light and blue light. The blue light (wavelength 360–450nm) can easily be switched on and off by the click of a button, giving doctors the benefit of both modes.
There are significant benefits of using BLC with Cysview® in addition to WLC alone:
- Better detection of papillary non-muscle invasive bladder cancer (NMIBC) due to the fluorescence of cancerous cells.1,2
- More complete tumour removal during resection by re-examining the resection area in blue light after resection in white light to see if there is any remnant tumour.2
- Improved surgical outcomes as a result of an enhanced detection and more complete resection that can lead to more-accurate risk categorization and better-informed treatment decisions.3
Improving the Patient Pathway
The typical patient pathway for an NMIBC patient is characterized by a cycle of follow-up cystoscopies and TURBTs. This ongoing series of examinations is what contributes to making bladder cancer one of the most expensive cancers to manage.4,5
Using BLC with Cysview®
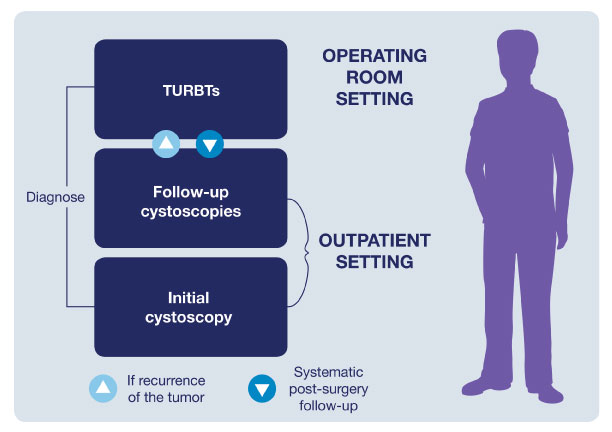
The correct use of BLC with Cysview is important to providing optimal treatment. The procedure is very similar to a standard WLC with some additional preparation:
1) Preparing and reconstituting Cysview.
In only a few steps the drug is reconstituted and ready
to be instilled in the patient's bladder.
See full description on how to reconstitute Cysview.1
If not administered shortly after reconstitution, the solution can be stored in the labelled syringe for up to 2 hours in a refrigerator at between 2 °C–8 °C. If not used within 2 hours, the solution must be discarded.

2) Instill Cysview. The Cysview solution is then instilled into the bladder. After bladder instillation, Cysview enters the bladder mucosa where it is converted to the photoactive porphyrin (PAP), mainly protoporphyrin IX (PpIX), which preferentially accumulates in rapidly proliferating neoplastic cells. After a period of only one hour, sufficient PAPs have been accumulated in neoplastic bladder tissue to allow BLC to take place. Under blue light Cysview causes lesions to appear bright red/pink and demarcated, whereas the background normal tissue appears dark blue.
See the full Cysview Bladder Instillation Procedure.1
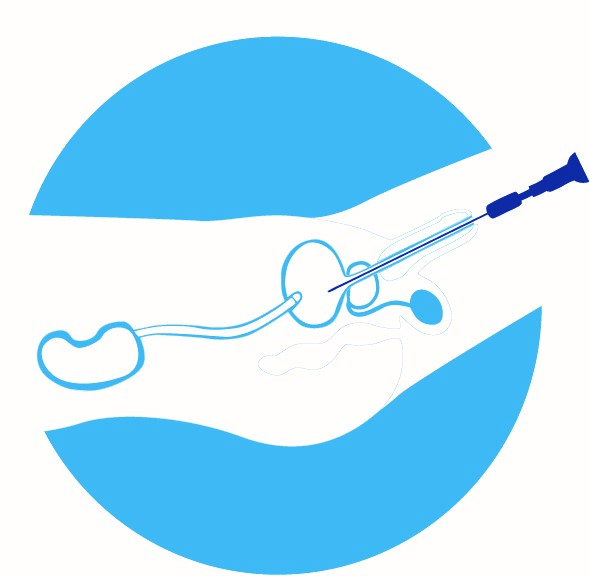
3) Examine bladder in both white and blue light.. A Blue Light Cystoscopy uses both white and blue light. The cystoscopic examination in blue light should be done within 1-3 hours of Cysview instillation. BLC with Cysview is superior to a WLC alone.

The recommended cystoscopy procedure is to:1
- Inspect the entire bladder in white light before switching to blue light mode and repeating the inspection. Make note of any lesions found.
- Remove any visible tumours in white light mode.
- Examine all areas in blue light mode to ensure that there are no tumour remnants left in the bladder.
- If necessary, repeat steps 2 and 3.
During the cystoscopic examination, be aware that:1
- Fluorescence is expected at the bladder outlet and the prostatic urethra; this fluorescence occurs in normal tissue and is usually less intense and more diffuse than the bladder mucosal fluorescence associated with malignant lesions.
- Tangential light may give false fluorescence. To help avoid false fluorescence, hold the endoscope perpendicular and close to the bladder wall with the bladder distended.
- False-positive fluorescence may result from scope trauma from a previous cystoscopic examination and/or bladder inflammation.
- Malignant lesions may not fluoresce following Cysview administration, particularly if the lesions are coated with necrotic tissue. Blue light may fail to detect T2 tumours that have a tendency to be necrotic on the surface, and necrotic cells generally do not fluoresce.
- When performing the BLC, avoid prolonged blue-light exposure. Studies have not evaluated the potential for adverse effects from blue light.
- Perform biopsy and/or resection of suspicious lesions by transurethral resection of the bladder (TURB) only after completing white- and blue-light cystoscopic examinations with bladder mapping. Using standard cystoscopic practices, obtain biopsies of abnormal areas identified during either white- or blue-light examination and perform resections in white light. Always check for the completeness of the resections under both white light and blue light before finalizing the TURB procedure.
Indication for Cysview® (hexaminolevulinate HCl)
Cysview is indicated as an adjunct to White Light Cystoscopy in the detection of non-muscle invasive papillary bladder
cancer in patients with known or suspected bladder cancer.
Only approved cystoscopic equipment should be used, equipped with necessary filters to allow both White Light Cystoscopy
(WLC) and Blue Light (wavelength 360–450nm) fluorescence Cystoscopy (BLC®). Training in Blue Light Cystoscopy
with an approved Photodynamic Diagnosis (PDD) System is essential prior to the use of Cysview.
For additional information about Cysview, please refer to the product monograph.
Note: Cysview (hexaminolevulinate (HAL) HCl) is used with Blue Light Cystoscopy (BLC) as an adjunct to White Light Cystoscopy (WLC) and not separately. Cysview with BLC may be referred to in a number of ways, such as Cysview, Cysview with BLC, Cysview with Photodynamic Diagnosis (PDD) System, HAL with BLC and HAL with PDD.
- Cysview® Canada Product Monograph. Jan 11, 2022.
- Hermann GG, Mogensen K, Carlsson S, et al. Fluorescence-Guided Transurethral Resection of Bladder Tumours Reduces Bladder Tumour Recurrence Due to Less Residual Tumour Tissue in Ta/T1 Patients: A Randomized Two-Centre Study. BJU Int. 2011;108(8b):E297-E303.
- Richards KA, Smith ND, Steinberg GD. The Importance of Transurethral Resection of Bladder Tumor in the Management of Nonmuscle Invasive Bladder Cancer: A Systematic Review of Novel Technologies. J Urol. 2014;191(6):1655-1664.
- Sievert KD, Amend B, Nagele U, et al. Economic Aspects of Bladder Cancer: What Are the Benefits and Costs? World J Urol. 2009; 27(3):295.
- Witjes JA, Redorta JP, Jacqmin D, et al. Hexaminolevulinate-Guided Fluorescence Cystoscopy in the Diagnosis and Follow-Up of Patients with Non-Muscle Invasive Bladder Cancer: Review of the Evidence and Recommendations. Eur Urol. 2010;57(4):607-614.

 contact
contact